The Research & Development of DeflaGyn® vaginal gel
At the turn of the millennium the antioxidant properties of several plant and mineral substances have been investigated. In collaboration with a German research team, a way was found to improve the usually low antioxidant capacity of selenite by mixing it with selective organic acids. It was known that Selenium (e.g. as selenites and selenates) is converted to selenoproteins in the human organism, e.g. to
- glutathione peroxidases (mitochondria, cytosol, intestinal cells, biomembranes, sperms)
- thioredoxin reductase (cell growth)
- deiodinases (thyroxine ➔ triiodothyronine)
and is therefore an essential trace element.
It was not known that selenium (selenite) in vitro in the acidic milieu becomes highly antioxidative. This method led to a change in the reduction potential from + 366mV (for pure selenite) to – 740mV (selenite, combined with citric acid) and thus to an enormous increase in anti-inflammatory effects.
Additional in vitro tests were able to prove the high antioxidant potential of this combination, which was henceforth labeled as DEFLAMIN®.
It should be noted that these antioxidant effects only come into their own when used externally. In the case of systemic application, the antioxidant effect is lost by increasing the pH value in the physiological environment (pH = 7.2 – 7.4).
Standard redox potentials of some nutrients
| E0 (Volt) | System |
|---|---|
| + 0.82 | O2 / H2O |
| + 0.366 (alkaline milieu) | Selenite |
| + 0.300 | Tocopherol (Vitamin E) |
| + 0.100 | Ubiquinone (Coenzyme Q10) |
| + 0.08 | Ascorbic acid (Vitamin C) |
| + 0 (+ 0.16 to – 0.02 V) | Flavonoids |
| – 0.12 | Riboflavin (Vitamin B2) |
| – 0.22 | Cystine / cysteine |
| – 0.23 | G SH / GSSG |
| – 0.29 | Thioctic acid (alpha lipoic acid) |
| – 0.32 | NADH + H+ / NAD+ |
| – 0.740 (acidic milieu) | Selenite |
Standard redox potentials of some nutrients
Redox potentials in volt of some important redox substances, measured against a hydrogen electrode. The electron transitions from plus to minus areas result in a voltage-dependent cascade or hierarchy. Vitamin E as a
pure radical scavenger would lie above the ubiquinone and would be regenerated by it. Coenzyme Q10 would be regenerated by vitamin C, this by flavonoids, this in turn by vitamin B2 and so on.
N. Fuchs, Mit Nährstoffen heilen, 4. Auflage, 2012.
Silicon Dioxide (SiO2)
Silicon dioxide has been known as an inert active ingredient in pharmaceutical and cosmetic applications for decades. According to its inertia, silicon dioxide has negligible toxicity. A second feature of silicon dioxide is its high adsorptive property, depending on the degree of comminution. The smaller the particle size, the greater the adsorptive capacity.
These two properties, chemical inactivity, and adsorptive properties, make silicon dioxide an interesting active ingredient with a high benefit-risk ratio for various applications.
From a medical point of view, silicon dioxide is often used as an adsorptive agent in liquids, gels or ointments for the adsorption and binding of bacteria, fungi, viruses. Due to its chemical inactivity, no pharmacological, metabolic, or immunological properties of silicon dioxide are described.
DeflaGyn® vaginal gel - Mechanism of Action
The mechanism of action is based both on the adsorbing properties of SiO2 and on the anti-oxidative effect of DEFLAMIN® (sodium selenite and citric acid). Adsorption of viruses to surfaces or particles is a process that is influenced by different biochemical and biophysical parameters and is subject to intense research since many years. Numerous publications
confirm the adsorptive binding of proteins, lipids and lipoproteins, viruses and bacteria by silicon dioxide.
Murray JP, Laband SJ. Degradation of poliovirus by adsorption on inorganic surfaces. Appl Environ Microbiol. (1979) 37:480–6. doi: 10.1128/AEM.37.3.480-486.1979
Barrett EG, Johnston C, Oberdorster G, Finkelstein, JN. Silica binds serum proteins resulting in a shift of the dose-response for silica-induced chemokine expression in an alveolar type II cell line. Toxicol Appl Pharmacol. (1999) 161:111–22. doi: 10.1006/taap. 1999.8793
Adsorption of Pathogens
The objective of the investigation was to demonstrate the adsorptive binding of bacteria / pathogenic agents to particles of a preparation containing silica gel using fluorescent microscopy.
The tests carried out with Staphylococcus aureus are well documented. The performed tests show quite clearly that it is only in the presence of silica that clearly defined particles form and which adsorb the investigated Staphylococcus aureus bacterium.
Highly dispersed micronized silicon dioxide (SiO2) particles adsorb pathogens (bacteria, viruses, fungi, cell residues, irritating particles).
Adsorption behavior of bacteria on preparations. Hahn A, Report ZetA Partikelanalytik GmbH, 2013.
Adsorption of viruses to charge-modified silica. Zerda KS, Appl Environ Microbiol. 1985 January;49(1):91-95.
Binding of Pathogens
An experiment was performed to detect the binding of bovine serum albumin (BSA) in a model vaginal fluid onto the silicon dioxide contained in DeflaGyn® vaginal gel.
This laboratory in vitro investigation has demonstrated direct evidence concerning the mechanism of action of DeflaGyn® vaginal gel using a vaginal secretion model test system by Prof. Geoffrey Lee / Friedrich-Alexander-Universität Erlangen.
The silicon dioxide contained in DeflaGyn® vaginal gel completely binds the model protein. By removing the SiO2 from DeflaGyn® vaginal gel the ability to bind BSA has evidently been lost.
This demonstrates the plausibility of the binding of organic molecules from the vaginal secretion to a single dose of DeflaGyn® vaginal gel.
Investigation of the Binding Properties of DeflaGyn® vaginal gel. Lee G 08 2013.
Determination of the binding properties of DeflaGyn® vaginal gel. Lee G 12 2013.
Owen D, Katz D, A vaginal fluid stimulant. Contraception 59: 91 – 95 (1999).
Neutralisation of Pathogens (Antioxidative- Antiinflammatory Effect)
Aim of the analysis was the evaluation of the antioxidant capacity of the product DeflaGyn® vaginal gel. In order to assess the antioxidant capacity, the ORAC assay (oxygen radical absorbance capacity) was applied. This method is widely used for food and cosmetics and has been standardized by Prior et al. 2005.
This series of assays indicates, that DEFLAMIN® or sodium selenite and citric acid contributes to the antioxidative capacity of the product. This is in concordance with the data of Albrecht et al. (1999), who found an antioxidative capacity of sodium selenite under physiological conditions.
Evaluating of the antioxidant capacity of the product „Vaginalgel“. Dr. Zimmermann, Institute Prof. Kurz (2013).
Albrecht, S., Zimmermann, T., Grützmann, R. et al. Zum redoxsensitiven Verhalten von Selenit in Gegenwart reaktiver Sauerstoffspezies. Med Klin 94, 70–73 (1999). https://doi.org/10.1007/BF03042197
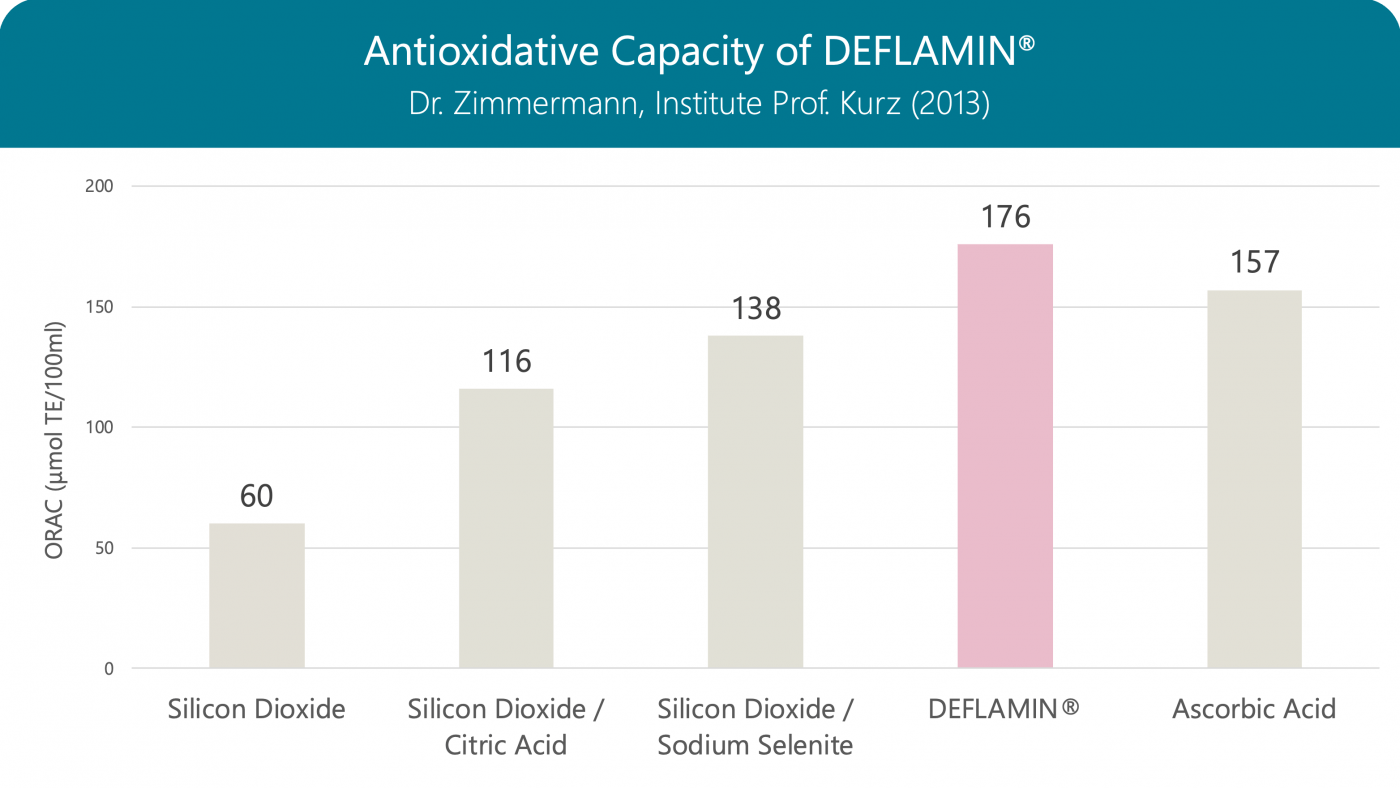
Antioxidative Capacity of DEFLAMIN®
The antioxidative capacity of DEFLAMIN® (a globally patented formula) is stronger than that of the individual components, and even stronger than that of vitamin C. Oxidative stress induced by infections and inflammation has an important role in DNA damage and cervical tumorigenesis. Studies suggest that oxidative stress likely plays a major role in the process of HPV DNA integration, which is an important step for malignant transformation of the cervical epithelium.
Oxygen Radical Absorbance Capacity (ORAC): When measuring, the vitamin E derivative Trolox serves as a reference, so the result is given in Trolox equivalents (TE)
Clinical Development
To prove the effectiveness of DeflaGyn® vaginal gel, a study was first carried out in Austria (Huber J et al. 2016).
The remission rate (improvement of the findings) of untreated patients was compared with the remission rate of patients treated with the DeflaGyn® vaginal gel over an observation period of 3‑4 months.
In 77% of patients with the baseline PAP III (ASC‑US, ASC‑H) and in 71% of patients with the baseline PAP IIID (LSIL, HSIL), improvement occurred after treatment with DeflaGyn® vaginal gel after 3‑4 months (PAP II).
The results of another, in this case randomized, open-label multicenter study on the therapeutic efficacy of the medical device DeflaGyn® vaginal gel in cervical lesions have confirmed, even far exceeded, these results (Major AL et al. 2020).
At comparable remission and regression rates (72.2%), as already described, the antiviral effect on high‑risk HPV (hr‑HPV clearance 54%) and beyond that an overly significant reduction in the oncogenic risk of the transformed cells could be demonstrated (p16/Ki‑67 negativity 83%).
Huber J et al.

Univ. Prof. DDr. Johannes C. Huber, Medical University of Vienna, University Clinic for Gynecology, Clinical Department for Gynecological Endocrinology and Reproductive Medicine
Routine Treatment of Cervical Cytological Cell Changes
Diagnostic Standard, Prevention and Routine Treatment of Cervical Cytological Cell Changes – An Assessment of Primary and Secondary Prevention and Routine Treatment Data in the Context of an Anonymous Data Collection from Practicing Gynaecologists; an Academic, Non-Interventional Study
Principal Investigators
J. Huber, B. Pötsch, M. Gantschacher, M. Templ
Gynecological application of DEFLAMIN® & highly dispersed SiO2 as DeflaGyn® vaginal gel
Project conducted by the academic, independent study network ANISNet (Academic Non-Interventional Study Net) of the SFU (Sigmund Freud Private University), Vienna, Austria.
Outcome
DeflaGyn® vaginal gel improves the regression of PAP III & PAP IIID significantly
Diagnosis and treatment of vaginal and cervical cytological cell changes are described in European and national guidelines. The aim of this data collection was to evaluate the remission rates of PAP III and PAP IIID cytological findings in patients over a period of 3‑4 months.
A new treatment option was used in gynecological practice on patients with PAP III and PAP IIID findings between confirmation and the next follow‑up with excellent success.
PAP classification and Bethesda equivalents, ÖGZ 2005, Breitenecker et al. Gyn Aktiv 3:16-20, 2005. PAP III (ASC‑US, ASC‑H), PAP IIID (LSIL, HSIL)
Major AL et al.

Univ. Prof. DDDr. Attila L. Major, Department of Obstetrics & Gynecology, Fribourg Cantonal Hospital, Switzerland, Head Physician at Femina Gynaecology Centre, Geneva, Switzerland
Post‑Market Clinical Follow‑up
Efficacy and safety of an adsorbent and anti‑oxidative vaginal gel on CIN1 and 2, on high‑risk HPV, and on p16/Ki‑67: a randomized controlled trial
Principal Investigators
A. Major, V. Dvořák, A. Skřivánek, T. Malík, M. Pluta
Gynecological application of DeflaGyn® vaginal gel
The effect of DeflaGyn® vaginal gel on histologically-proven cervical intraepithelial neoplasia type 2 (CIN2) as well as p16 positive CIN1, and on the presence of the onco-marker p16 was investigated.
Outcome Primary Endpoint
-
Endpoint: Cytological or histological Regression after 3 months
-
Result: 72.2% Active Arm vs. 25.0% Control Arm (p<0.001)
Outcome Secondary Endpoints
-
Significant cytological Regression after 3 months
-
Persistence of cytological Regression after 6 months
-
Significant clearance of oncogenic HPV-strains after 3 months
-
Significant decrease of CINtec® PLUS-positivity after 3 and 6 months
This prospective, open, two-arm, controlled, multicenter trial comparing the efficacy of DeflaGyn® vaginal gel with a non-treated control arm demonstrated that the medical device DeflaGyn® vaginal gel is effective for enhancing the regression of cervical lesions and preventing their progression.
The significant change in p16/Ki‑67 shows that DeflaGyn® vaginal gel is influencing oncogenic progress substantially, indicating that the DeflaGyn® vaginal gel is a potential therapy regimen for patients with HPV infected cervical lesions.
Cytology – BD SurePath™; HPV – cobas® 4800; p16/Ki‑67 – CINtec® PLUS
Scientific Publications on DeflaGyn® vaginal gel
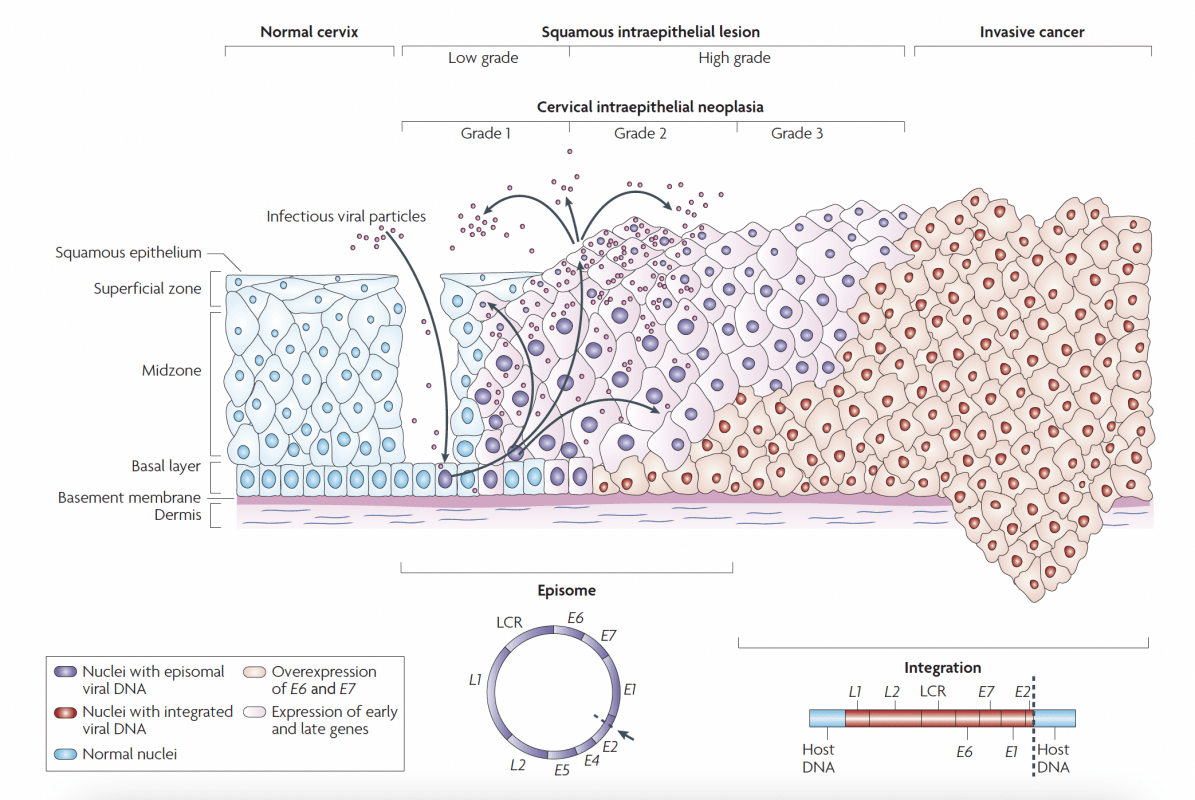
HPV Lifecycle
p16 plays an important role in the regulation of cell division. When p16 is expressed, a protein complex is formed from the retinoblastoma protein (pRb) and the transcription factor (E2F), which initiates cell cycle arrest. This means that p16 has an anti-proliferative effect under physiological conditions.
In oncogenically transformed cells, after oncogenic transformation by high‑risk HPV, the anti-proliferative effect of p16 is abolished. This leads to uncontrolled cell division, genetic instability and, at the same time, overexpression of p16.
After reproduction by host cells infectious viral particles are released by the cells of the cervical epithelium. Therefore, viral particles can be found in the vaginal secretion.
HPV-mediated progression to cervical cancer
Basal cells in the cervical epithelium rest on the basement membrane, which is supported by the dermis. Human papillomavirus (HPV) is thought to access the basal cells through micro-abrasions in the cervical epithelium.
Following infection, the early HPV genes E1, E2, E4, E5, E6 and E7 are expressed and the viral DNA replicates from episomal DNA (purple nuclei).
In the upper layers of epithelium (the midzone and superficial zone) the viral genome is replicated further, and the late genes L1 and L2, and E4 are expressed. L1 and L2 encapsidate the viral genomes to form progeny virions in the nucleus. The shed virus can then initiate a new infection.
Lowgrade intraepithelial lesions support productive viral replication. An unknown number of high-risk HPV infections progress to high-grade cervical intraepithelial neoplasia (HGCIN).
The progression of untreated lesions to microinvasive and invasive cancer is associated with the integration of the HPV genome into the host chromosomes (red nuclei), with associated loss or disruption of E2, and subsequent upregulation of E6 and E7 oncogene expression. LCR, long control region.
Human papillomavirus lifecycle and organization of its genome. Page 892. Crosbie EJ et al. Human papillomavirus and cervical cancer. Lancet 2013 September 7; 382:889-99.